2 Answers
1. **Synthesis (Combination) Reaction**:
- **Definition**: Two or more simple substances combine to form a more complex product.
- **General Form**: \( A + B \rightarrow AB \)
- **Example**: 2H₂ + O₂ → 2H₂O (Hydrogen and oxygen combine to form water).
2. **Decomposition Reaction**:
- **Definition**: A complex molecule breaks down into simpler substances.
- **General Form**: \( AB \rightarrow A + B \)
- **Example**: 2H₂O → 2H₂ + O₂ (Water decomposes into hydrogen and oxygen gases).
3. **Single Replacement (Displacement) Reaction**:
- **Definition**: One element replaces another in a compound.
- **General Form**: \( A + BC \rightarrow AC + B \)
- **Example**: Zn + 2HCl → ZnCl₂ + H₂ (Zinc replaces hydrogen in hydrochloric acid).
4. **Double Replacement (Metathesis) Reaction**:
- **Definition**: The ions in two compounds exchange places to form two new compounds.
- **General Form**: \( AB + CD \rightarrow AD + CB \)
- **Example**: AgNO₃ + NaCl → AgCl + NaNO₃ (Silver nitrate reacts with sodium chloride to form silver chloride and sodium nitrate).
5. **Combustion Reaction**:
- **Definition**: A substance reacts with oxygen, releasing energy in the form of light or heat.
- **General Form**: \( C_xH_y + O₂ \rightarrow CO₂ + H₂O \)
- **Example**: CH₄ + 2O₂ → CO₂ + 2H₂O (Methane burns in oxygen to produce carbon dioxide and water).
6. **Neutralization Reaction**:
- **Definition**: An acid reacts with a base to produce water and a salt.
- **General Form**: \( HA + BOH \rightarrow H₂O + AB \)
- **Example**: HCl + NaOH → NaCl + H₂O (Hydrochloric acid reacts with sodium hydroxide to form sodium chloride and water).
7. **Oxidation-Reduction (Redox) Reaction**:
- **Definition**: Involves the transfer of electrons between substances. One substance is oxidized (loses electrons) and another is reduced (gains electrons).
- **General Form**: Varies; involves changes in oxidation states.
- **Example**: 2Na + Cl₂ → 2NaCl (Sodium is oxidized, chlorine is reduced).
8. **Precipitation Reaction**:
- **Definition**: Two soluble salts react in solution to form an insoluble product (precipitate).
- **General Form**: \( AB_{(aq)} + CD_{(aq)} \rightarrow AD_{(s)} + CB_{(aq)} \)
- **Example**: Pb(NO₃)₂ + 2KI → PbI₂ (s) + 2KNO₃ (Lead nitrate reacts with potassium iodide to form lead iodide precipitate).
9. **Complexation Reaction**:
- **Definition**: A reaction where a complex ion is formed from a metal ion and ligands.
- **General Form**: Varies; typically involves metal ions and ligands forming a complex.
- **Example**: [Fe(CN)₆]⁴⁻ + 6K⁺ → K₆[Fe(CN)₆] (Formation of potassium hexacyanoferrate).
10. **Hydrolysis Reaction**:
- **Definition**: A compound reacts with water, often resulting in the breakdown of the compound.
- **General Form**: \( AB + H₂O \rightarrow AOH + BH \)
- **Example**: Na₂CO₃ + H₂O → 2NaOH + CO₂ (Sodium carbonate reacts with water).
11. **Photochemical Reaction**:
- **Definition**: A reaction triggered by light energy.
- **General Form**: Depends on the reaction; involves light as a reactant.
- **Example**: 2AgBr → 2Ag + Br₂ (Silver bromide decomposes into silver and bromine upon exposure to light).
12. **Electrolytic Reaction**:
- **Definition**: A reaction driven by electrical current, often used in electrolysis.
- **General Form**: Varies; involves decomposition or synthesis driven by electricity.
- **Example**: 2H₂O → 2H₂ + O₂ (Water is electrolyzed to produce hydrogen and oxygen gases).
13. **Combustion Reaction**:
- **Definition**: Similar to combustion, but often refers specifically to reactions involving organic compounds in oxygen.
- **General Form**: C_xH_y + O₂ → CO₂ + H₂O
- **Example**: C₅H₁₂ + 8O₂ → 5CO₂ + 6H₂O (Pentane burns in oxygen to produce carbon dioxide and water).
These reactions cover a wide range of chemical processes, illustrating how substances interact and transform in different scenarios.

Applied Physics

Signals and Systems

Digital Electronics
Basic Concepts
Basic Laws
Units

Ohmic Resistors
Capacitors and Inductors

RC Circuit
First-Order Circuits
Second-Order Circuits
Principles Of Circuit Analysis
Sinusoids and Phasors
AC Steady-State Analysis
Single Phase A.C. Circuits
Three-Phase Circuits
Resonance In Series And Parallel Circuits
Network Theorems
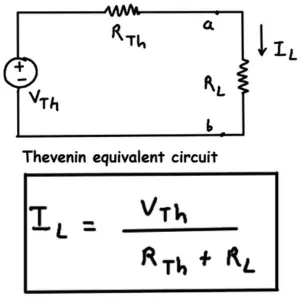
Thevenin's Theorem
Two-port Networks
Digital Electronics
Oscilloscope
Ohmmeter
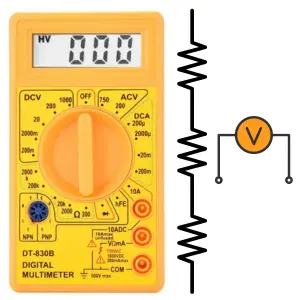
Voltmeter
Ammeter

Induction Motor
Transformer
Operational Amplifiers
Components
Symbols
Formulas
EE Notes
EE Dictionary
MCQ Quiz

Interview Q&A
Power Electronics Book
Advanced Calculator

Basic Calculator
Simulator
Videos
Q&A

Capacitance Meter
Two Way Switch
Electrical Machines
Power Electronics

Electrical Drives & Their Control

Electrical Safety & Standards

Basics of Electronics Engineering
Electromagnetic Fields

Electrical Machines

More Items Coming Soon...