2 Answers
### Pure Semiconductors (Intrinsic Semiconductors)
A **pure semiconductor** is one without any impurities. In its natural state, every atom in a pure semiconductor, like silicon or germanium, is bonded to four other atoms, creating a stable structure. At room temperature, some of these bonds may break due to heat, freeing up a few electrons to move and carry electric current.
In a pure semiconductor:
- **Electrons** (negatively charged particles) and **holes** (the absence of an electron, acting as a positive charge) are present in equal numbers.
- The ability of a pure semiconductor to conduct electricity is very low because very few electrons are available to carry the current.
### Doped Semiconductors (Extrinsic Semiconductors)
To improve the conductivity, we introduce impurities into a pure semiconductor. This process is called **doping**. Doped semiconductors are called **extrinsic semiconductors**. There are two types of doping:
#### 1. **N-type Semiconductor (Negative Type)**
In this type, an impurity with more electrons than the semiconductor (usually phosphorus or arsenic, which have 5 outer electrons) is added. This extra electron becomes free to move and conduct electricity, increasing the material's conductivity.
- **Example**: If we add phosphorus (P) to silicon (Si), phosphorus has 5 electrons in its outer shell, while silicon has 4. Phosphorus can only bond with 4 silicon atoms, leaving the 5th electron free to move around. This free electron enhances the conductivity.
- **Result**: The semiconductor has more **free electrons** than holes, making electrons the primary charge carriers.
#### 2. **P-type Semiconductor (Positive Type)**
Here, an impurity with fewer electrons than the semiconductor (usually boron or gallium, which have 3 outer electrons) is added. This creates a "hole" where an electron is missing. These holes act like positive charges that can move and allow current to flow.
- **Example**: If boron (B) is added to silicon, boron has only 3 electrons in its outer shell, while silicon has 4. As a result, one bond with silicon will have a "hole," or missing electron.
- **Result**: The semiconductor has more **holes** than free electrons, making holes the primary charge carriers.
### Summary of Differences:
- **Pure (Intrinsic) Semiconductors**: Equal number of electrons and holes, low conductivity.
- **Doped (Extrinsic) Semiconductors**: Conductivity is increased by adding impurities.
- **N-type**: More electrons, electrons are the majority carriers.
- **P-type**: More holes, holes are the majority carriers.
By combining **N-type** and **P-type** semiconductors in devices like diodes and transistors, we can control the flow of electricity in electronics, making modern technology possible.
### Pure Semiconductors: The Natural State
Let's start with a **pure semiconductor**, often made from silicon. Think of silicon atoms as people holding hands in a neat grid-like formation. In this grid, every atom shares electrons with its neighbors, creating stable bonds. But, there's something missing here for electricity to flow—free electrons. In a pure semiconductor, almost all the electrons are locked in bonds, so they can't move freely, which means no electricity flows easily. This is the natural state of semiconductors: they can conduct electricity, but only under special conditions, like when they're heated.
### Doped Semiconductors: Adding Some Spice
Now, to make semiconductors more useful, we **"dope"** them, which is like adding a bit of seasoning to a bland dish. This process involves adding tiny amounts of other elements to the silicon.
There are two ways we can dope a semiconductor:
1. **N-type (Negative-type) Semiconductor:**
Imagine adding a few atoms of phosphorus (which has five electrons to share) into the silicon grid. Phosphorus brings an extra electron to the party. Since these extra electrons aren’t tightly bound to the atoms, they’re free to move around. This allows electricity to flow more easily. In this case, the extra electrons are like extra players on a soccer field, ready to move whenever needed.
2. **P-type (Positive-type) Semiconductor:**
On the other hand, if we add boron (which has only three electrons to share) into the silicon, we create **holes** where an electron should be. These holes are like empty seats in a theater—electrons from nearby atoms want to jump into these seats. This movement of electrons to fill the holes creates the flow of electricity, even though it's really the electrons that are moving. So, instead of extra players, we now have vacant spots that attract players (electrons).
### The Magic of Combining N-type and P-type
When we combine **N-type** and **P-type** semiconductors together, we create something called a **PN junction**. This is like setting up a dam where water (or electricity, in our case) can only flow in one direction. This combination is crucial for making devices like diodes, transistors, and solar cells work.
In summary, pure semiconductors are like a still lake with no free movement of electrons, while doped semiconductors (either N-type or P-type) are like rivers where electrons or holes can flow, making the material more useful for conducting electricity. This controlled flow of electrons forms the basis for almost every electronic device we use today.

Applied Physics

Signals and Systems

Digital Electronics
Basic Concepts
Basic Laws
Units

Ohmic Resistors
Capacitors and Inductors

RC Circuit
First-Order Circuits
Second-Order Circuits
Principles Of Circuit Analysis
Sinusoids and Phasors
AC Steady-State Analysis
Single Phase A.C. Circuits
Three-Phase Circuits
Resonance In Series And Parallel Circuits
Network Theorems
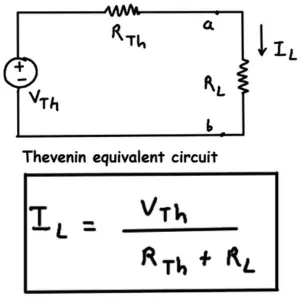
Thevenin's Theorem
Two-port Networks
Digital Electronics
Oscilloscope
Ohmmeter
Voltmeter
Ammeter

Induction Motor
Transformer
Operational Amplifiers
Components
Symbols
Formulas
EE Notes
EE Dictionary
MCQ Quiz

Interview Q&A
Power Electronics Book
Advanced Calculator

Basic Calculator
Simulator
Videos
Q&A

Capacitance Meter
Two Way Switch
Electrical Machines
Power Electronics

Electrical Drives & Their Control

Electrical Safety & Standards

Basics of Electronics Engineering
Electromagnetic Fields

Electrical Machines

More Items Coming Soon...