1 Answer
Here is a list of 22 states of matter, beyond the classical five:
1. **Solid**: Particles are closely packed in a fixed arrangement, and they vibrate but don’t move past one another.
2. **Liquid**: Particles are close but can move past each other, allowing the substance to flow and take the shape of its container.
3. **Gas**: Particles are far apart and move freely, filling any available space.
4. **Plasma**: High-energy gas-like state where atoms are ionized (electrons are freed from atoms), consisting of positive ions and free electrons.
5. **Bose-Einstein Condensate (BEC)**: Forms at temperatures close to absolute zero, where atoms coalesce into a single quantum state, behaving as a single "super atom."
6. **Fermionic Condensate**: Similar to BEC but composed of fermions (particles like electrons), it occurs at ultra-cold temperatures and has unique properties like pairing and superconductivity.
7. **Quark-Gluon Plasma**: This state exists at extremely high temperatures and energy levels, where quarks and gluons, the fundamental components of protons and neutrons, are no longer confined inside particles.
8. **Supersolid**: A phase that exhibits properties of both solids and superfluids, allowing it to flow without friction despite having a rigid structure.
9. **Superfluid**: A state of matter where liquid flows without viscosity and exhibits perfect fluidity, often at temperatures near absolute zero (e.g., helium-4).
10. **Supercritical Fluid**: A state that occurs when a substance is at a temperature and pressure above its critical point, exhibiting properties between liquids and gases.
11. **Rydberg Matter**: Consists of Rydberg atoms where electrons are excited to high-energy states; this state has potential applications in quantum computing.
12. **Photonic Matter**: In this state, light behaves like matter, which occurs under special conditions like in some types of optical lattices or with strong interactions between light and matter.
13. **Plasmonic States**: These involve collective oscillations of free electron gas in a metal or semiconductor and can be manipulated to carry energy or as energy converters in nanosystems.
14. **Chiral Matter**: A state where the chirality (handedness) of matter plays a significant role, affecting how particles interact. This state has been a subject of study in high-energy physics.
15. **Glass**: An amorphous solid with disordered atomic structure; it flows extremely slowly under stress but doesn’t have a defined melting point.
16. **Time Crystals**: These have a structure that repeats in time (rather than space), potentially opening up new possibilities in quantum computing and energy storage.
17. **Dropleton**: This state forms from electron-like particles in two-dimensional materials, with distinct behavior from solids, liquids, and gases.
18. **Bose Glass**: A disordered phase where a Bose-Einstein condensate encounters obstacles in its formation, leading to a non-ordered arrangement.
19. **Strange Matter**: Composed of quarks, specifically "strange quarks," it may form in the cores of neutron stars and might have unique properties from regular nuclear matter.
20. **Quantum Foam**: At the Planck scale (the smallest scale in the universe), spacetime itself is expected to be turbulent, creating microscopic bubbles and disturbances.
21. **Excitonium**: A newly identified phase where a new type of quantum matter (excitons, which are pairs of electrons and holes) may condense to form a stable state.
22. **Plasma Crystal**: A state where plasma behaves like a crystal, often seen in the laboratory conditions where ionized gas particles become ordered into a solid-like lattice structure.
Many of these states are theoretical or exist under specific experimental conditions. As research progresses, new states of matter may be discovered, expanding our understanding of how matter behaves under extreme conditions.

Applied Physics

Signals and Systems

Digital Electronics
Basic Concepts
Basic Laws
Units

Ohmic Resistors
Capacitors and Inductors

RC Circuit
First-Order Circuits
Second-Order Circuits
Principles Of Circuit Analysis
Sinusoids and Phasors
AC Steady-State Analysis
Single Phase A.C. Circuits
Three-Phase Circuits
Resonance In Series And Parallel Circuits
Network Theorems
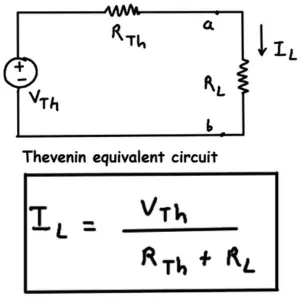
Thevenin's Theorem
Two-port Networks
Digital Electronics
Oscilloscope
Ohmmeter
Voltmeter
Ammeter

Induction Motor
Transformer
Operational Amplifiers
Components
Symbols
Formulas
EE Notes
EE Dictionary
MCQ Quiz

Interview Q&A
Power Electronics Book
Advanced Calculator

Basic Calculator
Simulator
Videos
Q&A

Capacitance Meter
Two Way Switch
Electrical Machines
Power Electronics

Electrical Drives & Their Control

Electrical Safety & Standards

Basics of Electronics Engineering
Electromagnetic Fields

Electrical Machines

More Items Coming Soon...